Abstract
Introduction: Carfilzomib (CFZ) is an epoxyketone-based irreversible proteasome inhibitor that is one of the leading treatments for multiple myeloma (MM). Cardiotoxicity is the primary limitation for the clinical use of CFZ. We developed a novel liposome nanoparticle formulation of CFZ, a compound with low water solubility (<5 µg/mL) and low pKa (5.14) (LS-CFZ) that dramatically reduces the toxicity, increases the plasma half-life, and improves the antitumor efficacy compared to the commercial formulation. In addition, CFZ was combined in the same liposome, with vinblastine (VBL) an autophagy inhibitor. The combination exhibited synergistic anti-tumor cell activity in vitro and in vivo using a colon cancer (C26) mouse tumor model.
Methods: CFZ was screened for cytotoxicity in combination with various cell lines including MM and C26 with a focus on autophagy inhibitors. VBL was co-loaded into the same liposomes with CFZ, and also loaded into separate particles. Formulations with various lipids, trapping agents and drug to lipid ratios were screened for drug loading efficiency. The lead formulation was selected based on mouse plasma half-life after IV injection in male CD1 mice. The plasma concentration of CFZ, VBL and a fluorescent lipid (liposome) label (DiIC18(5)) were measured by HPLC. The dose ratio of the CFZ and VBL was by determined by the MTD in tumor-free mice and the optimum dose and ratio was used for the efficacy study. The lead formulations were evaluated for anti-tumor efficacy in the C26 colon cancer using a single treatment (day 1) when the tumor volume was 200 mm3 for all liposome formulations and two treatments (day 1 and day 2) (optimum for CFZ) for the unencapsulated CFZ and VBL.
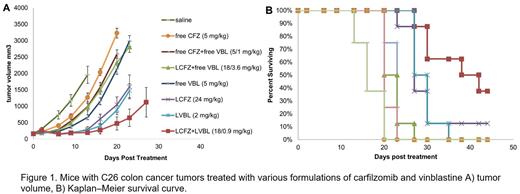
Results: Cytotoxicty studies in C26 cells indicated the optimum ratio of CFX/VBL was between 5:1 and 20:1. All liposomes used had a diameter of 110-130 nm. Ammonium sucrose octasulfate yielded the longest retention of CFZ and VBL in the liposome after IV injection in mice. The best lipid composition was sphingomyelin/Cholesterol/PEG-2000-PE (55/45/2.8, mol/mol/mol) with a particle size of 110-130 nm in diameter. Cryo TEM images of the LS-CFZ show a dense amorphous mass of drug precipitate in the center of the liposomes. The drug to lipid ratio chosen for use in the efficacy study was 101 g/mol lipid for CFZ and 103 g/mol lipid for VBL with a loading efficiency >90%. Using the optimized trapping agent and lipid composition the %ID of CFZ is nearly identical to that of the liposome carrier up to 6 h; an average of 98.4±5.4% of the drug is retained up to 6 h. The %ID of CFZ decreases below that of the liposome between 6 and 24 h and most (>98%) of the CFZ has been released from the liposomes by the 24 h point. The AUC for LS-CFZ (2 mg/kg) is 1584 fold higher than CFZ administered as SBCD-CFZ (sulfobutyl cyclodextrin formulated, Kyprolis®) (5 mg/kg). CFZ administered as SBCD-CFZ has a t1/2 of 0.2 h compared to 3.0 h for LS-CFZ. VBL had slightly better stability than CFZ with a plasma half-life of 4.9 h. LS-CFZ had single dose MTD of 30 mg/kg for L-CFZ, while SBCD-CFZ given QDx2 had an MTD 6 mg/kg. The dramatic decrease in toxicity is likely due to lower Cmax of the bioavailable CFZ and reduced cardiotoxicity. The increased tolerability of LS-CFZ allowed for a CFZ/VBL ratio of 20:1 (18/0.9 mg/kg, CFZ/VBL) for the liposome formulation; the unencapsulated CFZ and VBL were administered at a ratio of 5:1 (5/1 mg/kg, CFZ/VBL). The anti-tumor efficacy of LS-CFZ/VBL combination was far superior to all other treatments. LS-CFZ was nearly identical to LS-VBL, and both single agents had more activity than any unencapsulated drug. As a monotherapy, LS-CFZ was far more efficacious than SBCD-CFZ, which is commonly used for treatment of multiple myeloma patients.
Conclusion: Liposome formulation of CFZ increased the tolerability by 5-fold compared to the commercial CFZ formulation. The described liposome technology enables significant improvement in anti-tumor efficacy for CFZ as a monotherapy or in combination against a C26 mouse model. The improved activity in solid tumors warrants investigation of LS-CFZ for MM treatment.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal